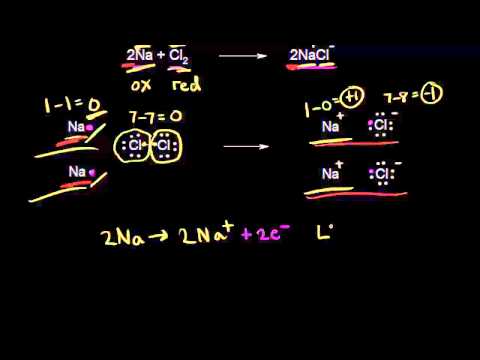
How to Find Oxidizing and Reducing Agents
Hydrogen is being oxidized from an oxidation state of 0 to 1 so it is the reducing agent. Note that while a specific atom typically has an odization state changes the agents are the actual species not the atoms.

Oxidizing And Reducing Agents Youtube
Strong reducing agents are weak oxidizing agents.
. A reducing agent can be oxidized by losing some of its electrons. In other words the sulfur is causing the zinc to be oxidized. If youre seeing this message it means were having trouble loading external resources on our website.
Fluorine chlorine iron etc. Hydrogen peroxide often one of the best choices for an oxidizing agent in the lab. Sodium hydrogen and lithium are examples of strong oxidizing agents.
Identify the oxidizing agent and reducing agent in the reactions. While weak reducing agents cannot lose electrons easily. MnO 4 SO 3 2 Mn 2 SO 4 2.
Sodium hydrogen and lithium are examples of strong oxidizing agents. Lets practice identifying oxidizing and reducing agents in redox reactions. Oxidation and reduction reactions play important roles in chemistry.
Hydrogen peroxide is the simplest compound having a peroxide functional group with an oxygen-oxygen single bond. Strong reducing agents are electropositive elements which can lose electrons easily in the chemical reactions. In the reaction above zinc is being oxidized by losing electrons.
Are weak reducing agents. The reaction takes place in basic aqueous solution. List of Oxidizing Agents.
If the oxidation number is greater in the product then it lost electrons and the substance was oxidized. Going from a 2 to a 3 or from a -2 to a -1 while reducing agents cause the other compounds oxidation number to. However there must be another substance present that gains those electrons and in this case that is the sulfur.
Hydrogen peroxide H 2 O 2 Potassium nitrate. Click to see full answer. Solution for To do.
Sodium or calcium hypochlorite very strong oxidizing agent. 2Na2S2O32 Na2S4O62Nal 22FeCl3H₂S2FeCl₂2S2HCI 33MgN₂Mg3N2. A reducing agent is a substance that causes another substance to reduce.
Because this oxidation state lies between the extremes of the more common 0 and -2 oxidation states of oxygen H 2 O 2 can act as either an oxidizing agent or a reducing agent. 4 aq is the oxidizing agent. So to identify an oxidizing agent simply look at the oxidation number of.
Identify the oxidizing and reducing agents in the skeletal unbalanced reaction. Then balance the reaction including the phase solid liquid etc of each species. Common examples of oxidizing agents are listed below.
It explains how to determine which reacta. B Nitrogen is being oxidized from an oxidation state of 0 to 1 so it is the reducing agent. Zn Zn 2 2 e Reduction.
Potassium dichromate be careful as the Cr 6 ion is carcinogenic. How To Find The Oxidizing Agent. S 2 e S 2.
A reducing agent reductant loses electrons and is oxidized in a chemical reaction. Als is the reducing agent. This chemistry video tutorial explains how to find the oxidizing agent and the reducing agent in a redox reaction.
SO 3 2 is the reducing agent and MnO 4 is the oxidizing agent. Many other oxidizing agents are commonly used industrially as well as in the day-to-day lives of humans. It is typically in one of its lower possible oxidation states and is known as an electron donor.
Oxidizing agents cause the other compounds oxidation number to increase ex. These reactions involve the loss of electrons in the case of oxidation or. Oxygen is being reduced from an oxidation state of 0 to -2 so it is the oxidizing agent.
So to identify an oxidizing agent simply look at the oxidation number of an atom before and after the reactionIf the oxidation number is greater in the product then it lost electrons and the substance was oxidized. So to identify an oxidizing agent simply look at the oxidation number of an atom before and after the reaction. This video tutorial shows you how to identify the oxidizing and reducing agent in a redox reaction.
A reducing agent reductant loses electrons and is oxidized in a chemical reaction. It finds its uses as a weak oxidizing agent disinfectant and a bleaching agent. Halogens such as chlorine and fluorine Oxygen.
H 2 g Cl 2 g 2 HCl g Another example is hydrogen peroxide in which the oxygen atom is in the -1 oxidation state. It has plenty of examples and practice problems for you.

Oxidizing And Reducing Agents Video Khan Academy
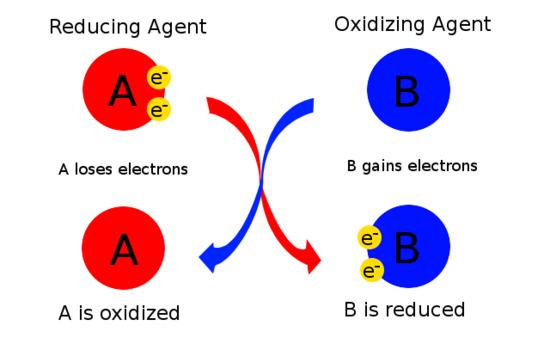
8 2 Oxidizing And Reducing Agents Chemistry Libretexts

Oxidation And Reduction Reactions Basic Introduction Youtube
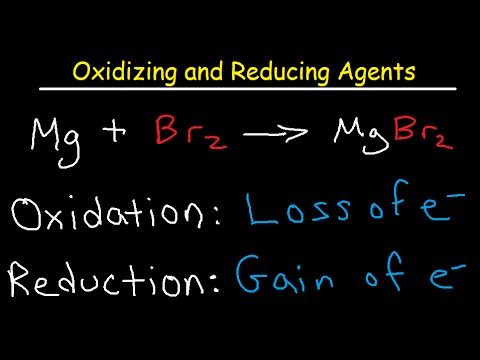
0 Response to "How to Find Oxidizing and Reducing Agents"
Post a Comment